Abstract
Background
The outcome of MDS patients (pts) is heterogeneous, with some pts living for years, while others die within a few months of diagnosis. Several prognostic models have been developed to risk stratify pts with MDS including: the International Prognostic Scoring System (IPSS), Revised IPSS (IPSS-R), World Health Organization classification-based Prognostic Scoring System (WPSS), and MD Anderson Prognostic Scoring System (MDPSS). When applying these models to clinical practice, considerable heterogeneity in their prediction of outcomes is observed. Understanding such heterogeneity can impact disease expectations and treatment recommendations, as these can differ considerably based on the model used.
Methods
Clinical data from pts who were diagnosed with MDS according to 2008 WHO criteria and treated at our institution between 1/2000 and 1/2017 were analyzed. All prognostic models were calculated at the time of MDS diagnosis. Overall survival (OS) was calculated from the time of diagnosis to time of last follow up or death. Descriptive statistics were used to study the changes in risk categories across models.
Results
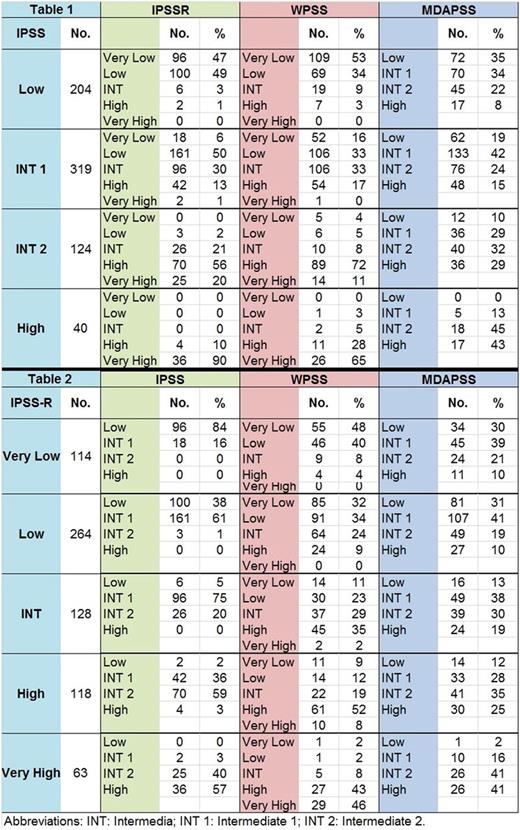
Of 687 pts included in the final analysis, the median age at diagnosis was 68 (11-99) years with a male (63%) predominance. Cytogenetic analysis per IPSS-R: very good 19 (3%), good 441(64%), intermediate (INT) 117 (17%), poor 47 (7%), and very poor 63 (9%). Risk categories by IPSS included: 204 (30%) with low risk, 319 (46%) intermediate-1 (INT-1), 124 (18%) intermediate-2 (INT-2), and 40 (6%) high risk. Risk categories by IPSS-R included: 114 pts (17%) with very low risk, 264 (38%) low, 128 (19%) intermediate, 118 (17%) high, and 63 (9%) very high.
Significant heterogeneity was observed when comparing all models to IPSS-R risk categories. Among 378 pts with lower-risk disease (Very Low / Low) per IPSS-R, 3 pts (1%) were re-classified INT-2 per IPSS, 73 (19%) as INT and 28 (7%) as high/very high per WPSS, and 11 (29%) as higher-risk per MDAPSS, Table 1. Among 128 pts with intermediate risk by IPSS-R, 102 (20%) were reclassified as lower-risk per IPSS, 44 (34%) as lower-risk and 47 (37%) as higher-risk per WPSS, and 65 (50%) as lower-risk per MDAPSS, Table 1. Among 181 pts with high/very high-risk by IPSS-R, 44 (25%) were reclassified as lower-risk by IPSS, 54 (30%) per WPSS, and 58 (32%) per MDAPSS, Table1. Among 523 pts with lower-risk (low / INT-1) by IPSS, 102 (20%) were reclassified as INT risk and 46 (9%) as higher-risk per IPSS-R, 113 (22%) as INT and 62 (12%) as higher-risk per WPSS, and 186 (36%) as higher risk per MDAPSS, Table 2. Among 164 pts with higher-risk (INT-2/High) per IPSS, 26 (16%) were reclassified as INT per IPSS-R, 24 (15%) per WPSS, and 53 (32%) per MDAPSS, Table 2.
The predicted OS per IPSS-R was overestimated when compared to the actual OS (evaluable in 429 pts) for pts in lower-risk categories. The difference between the median predicted IPSS-R OS and median actual OS was 70.6 months (m) for very low risk, 54 m for low, 9.5 m for INT, 6.7 m for high, and -2.4 m for very high risk (pts lived longer than their predicted OS). The IPSS, on the other hand, underestimated the OS, as the difference between the median predicted OS and median actual OS was -23.3 m for low risk, -16.5 m for INT1, -11.8 m for INT2, and -11.1 m for high risk.
Conclusions
Significant intra-patient (difference of outcome in the same pt based on the model used) and intra-group (difference in outcome among pts who have similar risk category by a given model) variability is observed when applying commonly used models in MDS. More importantly, IPSS-R overestimate OS for lower-risk disease and IPSS underestimate OS across all risk categories. This heterogeneity can impact survival expectations, treatment strategies, and clinical trial eligibility criteria based on the model used. A personalized prediction model that can precisely forecast the actual outcome of a pt is needed.
Gerds: Incyte: Consultancy; CTI BioPharma: Consultancy. Advani: Pfizer: Consultancy; Takeda/ Millenium: Research Funding. Maciejewski: Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fees; Apellis Pharmaceuticals: Consultancy; Ra Pharma: Consultancy. Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal